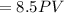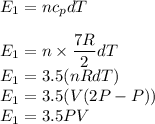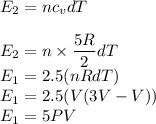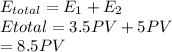Asample of a diatomic ideal gas has pressure p and volume v. when the gas is warmed, its pressure triples and its volume doubles. this warming process includes two steps, the first at constant pressure and the second at constant volume. determine the amount of energy transferred to the gas by heat. (here we define a "diatomic ideal gas" to havecv =52randcp =72r. use any variable or symbol stated above as necessary.)

Answers: 1


Other questions on the subject: Physics


Physics, 22.06.2019 11:20, puppylove899
Wave functions describe orbitals in a hydrogen atom. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 2: the quantum number l can have values from to . the total number of orbitals possible at the n = 2 energy level is .
Answers: 3

Physics, 22.06.2019 11:30, rwerjekrryery6750
In order of decreasing light-transmitting capabilities of materials, which is the correct sequence? a. transparent -> translucent -> opaque b. opaque -> transparent -> translucent c. opaque -> translucent -> transparent d. translucent -> transparent -> opaque
Answers: 1

Physics, 22.06.2019 15:20, ineedhelp2285
Aphoton is absorbed by an electron that is in the n = 3 state of a hydrogen atom, causing the hydrogen atom to become ionized. very far away from the nucleus, the released electron has a velocity of 750,000 m/s. what was the wavelength of the absorbed photon?
Answers: 2
You know the right answer?
Asample of a diatomic ideal gas has pressure p and volume v. when the gas is warmed, its pressure tr...
Questions in other subjects:



History, 12.09.2019 00:30