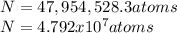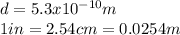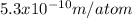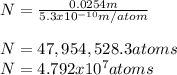Chemistry, 12.09.2019 00:30 inesperezferrere
Cesium atoms are the largest of the naturally occurring elements. they have a diameter of 5.30 1010 m. calculate the number of cesium atoms that would have to be lined up to give a row of cesium atoms 2.54 cm (1 in.) long.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Cesium atoms are the largest of the naturally occurring elements. they have a diameter of 5.30 1010...
Questions in other subjects:

Biology, 28.01.2020 21:03


Mathematics, 28.01.2020 21:03

Spanish, 28.01.2020 21:03



Business, 28.01.2020 21:03



History, 28.01.2020 21:03