Chemistry, 27.05.2020 19:01 laneycasey9058
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume of hydrogen gas under these conditions is 0.089 g. If both volumes contain the same number of gas particles (according to Avogadro's hypothesis), how can this difference in mass be explained?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1


Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
One liter of oxygen gas at standard temperature and pressure has a mass of 1.43 g. The same volume o...
Questions in other subjects:

Mathematics, 01.04.2021 16:00




English, 01.04.2021 16:00



English, 01.04.2021 16:00


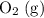 is larger than that of
is larger than that of 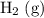 (by a factor of about
(by a factor of about 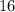 .) Therefore, the mass of the
.) Therefore, the mass of the 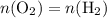 .
.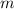 is different from the number of gas particles
is different from the number of gas particles 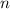 in it. In particular, if all particles in this gas have a molar mass of
in it. In particular, if all particles in this gas have a molar mass of 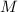 , then:
, then: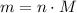 .
.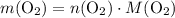 .
.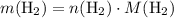 .
.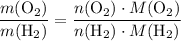 .
.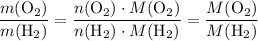 .
. :
: 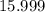 .
.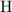 :
: 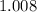 .
.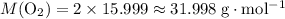 .
.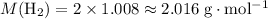 .
.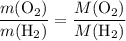 :
: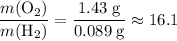 .Right-hand side:
.Right-hand side: 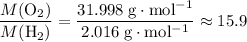 .
.