
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300 K. The temperature of the gas in the first cylinder is increased to 412 K at constant volume by doing work W1 and transferring energy Q1 by heat. The temperature of the gas in the second cylinder is increased to 412 K at constant pressure by doing work W2 while transferring energy Q2 by heat.
Required:
Find ÎEint, 1, Q1, and W1 for the process at constant volume.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 23:30, jude3412
Part a determine the magnitude of the x component of f using scalar notation. fx f x = nothing lb request answer part b determine the magnitude of the y component of f using scalar notation. fy f y = nothing lb request answer part c determine the magnitude of the z component of f using scalar notation. fz f z = nothing lb request answer provide feedback figure1 of 1a force vector acting on a ring attached to the ground is shown in the xyz space together with its x, y, and z components lying on the corresponding positive axes. the ring is located at the origin. force f is located in the first octant. f makes an angle of 60 degrees with its x component and an angle of 45 degrees with its y component. a force vector acting on a ring attached to the ground is shown in the xyz space together with its x, y, and z components lying on the corresponding positive axes. the ring is located at the origin. force f is located in the first octant. f makes an angle of 60 degrees with its x component and an angle of 45 degrees with its y component.
Answers: 2

Physics, 22.06.2019 04:30, jakiyahporter0817
Asystem containing an ideal gas at a constant pressure of 1.22×10^5 pa gains 2140 j of heat. during the process, the internal energy of the system increases by 2320 j. what is the change in volume of the gas?
Answers: 3

Physics, 22.06.2019 20:30, laylay2380
Atemperature inversion can cause a warm air mass to move over a colder air mass. this traps the colder air mass and prevents surface air from rising. this may result in which of these? a) smog b) acid rain c) freezing rain d) a thunderstorm
Answers: 3

Physics, 23.06.2019 00:00, elyssa34972
Examine the drawing of a molecule. each ring represents an atom of nitrogen (n). what’s the chemical formula of this substance?
Answers: 2
You know the right answer?
Two identical cylinders with a movable piston contain 0.7 mol of helium gas at a temperature of 300...
Questions in other subjects:


Mathematics, 09.10.2019 04:10


History, 09.10.2019 04:10

History, 09.10.2019 04:10

Mathematics, 09.10.2019 04:10




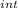 ,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J
,₁ = 977.7 J , Q₁ = 977.7 J and W₁ = 0 J  = 300 K, T
= 300 K, T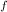 = 412 K, n = 0.7 mol
= 412 K, n = 0.7 mol 


