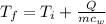Physics, 12.08.2020 18:01 valeriegarcia12
The same heat transfer into identical masses of different substances produces different temperature changes. Calculate the final temperature in degrees Celsius when 1.50 kcal of heat enters 1.50 kg of the following, originally at 15.0°C.(a) water
(b) concrete
(c) steel
(d) mercury

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 08:40, tasniahussain21
The system is released from rest with the cable taut, and the homogeneous cylinder does not slip on the rough incline. determine the angular acceleration of the cylinder and the minimum coeffi cient s of friction for which the cylinder will not slip.
Answers: 2

Physics, 22.06.2019 13:10, pearlvldz
The atoms in a nickel crystal vibrate as harmonic oscillators with an angular frequency of 2.3 × 1013 rad/s. the mass of a nickel atom is 9.75 × 10-26 kg. what is the difference in energy between adjacent vibrational energy levels of nickel? (h = 6.626 × 10-34 j • s, , 1 ev = 1.60 × 10-19 j)
Answers: 2

Physics, 22.06.2019 17:30, janicemaxwell123
Does heating a metal wire increase or decrease its electrical resistance? why?
Answers: 1

Physics, 22.06.2019 20:40, KekePonds1021
Aloop of wire is in a magnetic field such that its axis is parallel with the field direction. which of the following would result in an induced emf in the loop? choose all that apply. a loop of wire is in a magnetic field such that its axis is parallel with the field direction. which of the following would result in an induced emf in the loop? choose all that apply. moving the loop outside of the magnetic field region. spin the loop such that its axis does not consistently line up with the magnetic field direction. change the magnitude of the magnetic field. change the diameter of the loop
Answers: 1
You know the right answer?
The same heat transfer into identical masses of different substances produces different temperature...
Questions in other subjects:


Mathematics, 18.05.2021 21:40



Biology, 18.05.2021 21:40


Chemistry, 18.05.2021 21:40