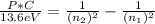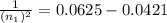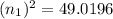Physics, 25.01.2020 06:31 brooke2828
Ahydrogen atom emits a photon that has momentum 0.3059×10^(-27) kg·m/s. this photon is emitted because the electron in the atom falls from a higher energy level into the n = 4 level. what is the quantum number of the level from which the electron falls? use values of h = 6.626×10^-34 j·s, c = 2.998×10^8 m/s, and e = 1.602×10^(-19) c.

Answers: 3


Other questions on the subject: Physics


Physics, 22.06.2019 05:00, DASASDAEDWEDA
Wavelength, frequency, and energy are related. what happens to a wave as it’s wavelength gets shorter?
Answers: 2

Physics, 22.06.2019 07:20, MannyBanko1350
If the ama of the inclined plane below is 2, calculate the ima and efficiency. ima = efficiency =
Answers: 1

Physics, 22.06.2019 11:30, joThompson
4. a 75.0 g piece of ag metal is heated to and dropped into 50.0 g of water at the final temperature of the mixture is what is the specific heat capacity of silver? 5. a 465 g chunk of iron is removed from petrucci, ralph h.. general chemistry (p. 290). pearson education. kindle edition.
Answers: 3
You know the right answer?
Ahydrogen atom emits a photon that has momentum 0.3059×10^(-27) kg·m/s. this photon is emitted becau...
Questions in other subjects:

Mathematics, 13.07.2019 15:10

Mathematics, 13.07.2019 15:10

Biology, 13.07.2019 15:10

Biology, 13.07.2019 15:10


Mathematics, 13.07.2019 15:10




History, 13.07.2019 15:10