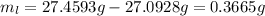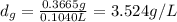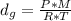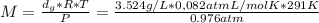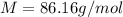Physics, 22.10.2019 23:00 mildredelizam
Asample of an unknown volatile liquid was injected into a dumas flask (mflask = 27.0928 g, vflask = 0.1040 l) and heated until no visible traces of the liquid could be found. the flask and its contents were then rapidly cooled and reweighed (mflask+vapor = 27.4593 g). the atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °c, respectively. the unknown volatile liquid was

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 07:30, crodriguez87
Clothes dryer uses about 7 amps of current from a 240 volt line. how much power does it use?
Answers: 1

Physics, 22.06.2019 15:20, alissalhenry
Arigid tank is divided into two equal parts by a partition. one part of the tank contains 3 kg of compressed liquid water at 400 kpa and 60°c while the other part is evacuated. the partition is now removed, and the water expands to fill the entire tank. determine the entropy change of water during this process, if the final pressure in the tank is 40 kpa. use steam tables.
Answers: 3

Physics, 22.06.2019 17:30, funnybugy16
How does the entropy of steam compare to the entropy of ice?
Answers: 2

Physics, 22.06.2019 21:30, tashaylinm02
Which sections of the heating curve illustrate this process?
Answers: 2
You know the right answer?
Asample of an unknown volatile liquid was injected into a dumas flask (mflask = 27.0928 g, vflask =...
Questions in other subjects:

Mathematics, 09.11.2020 22:40


History, 09.11.2020 22:40


Mathematics, 09.11.2020 22:40




Mathematics, 09.11.2020 22:40