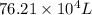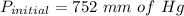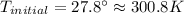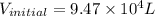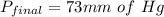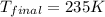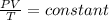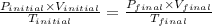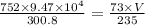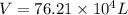Physics, 16.10.2019 22:00 wafflewarriormg
Ahelium-filled balloon is launched when the temperature at ground level is 27.8°c and the barometer reads 752 mmhg. if the balloon's volume at launch is 9.47 × 10 4 4 l, what is the volume in liters at a height of 36 km, where the pressure is 73.0 mm hg and temperature is 235.0 k?

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 21:20, mmaglaya1
Which of the following explains why it took so long for the public to accept the negative health effects of smoking? a)people were waiting for counter reasons, and those were presented. b)scientists had not completely finalized their results, which took 30 years. c)cigarettes were cheaply available and media channels were very inefficient. d)cigarette manufacturers were trying hard to bring an alternative into the market
Answers: 3

Physics, 22.06.2019 02:30, JanaMiqdad1003
Agas initially at p1 = 1 bar and occupying a volume of 0.5 liter is compressed within a piston–cylinder assembly to a final pressure p2 = 4 bar. (a) if the relationship between pressure and volume during the compression is pv = constant, determine the volume, in liters, at a pressure of 3 bar. (b) repeat for a linear pressure–volume relationship between the same end states. reference
Answers: 1

Physics, 22.06.2019 03:00, vinp190p9zekn
Which boundary is associated with the building of the himalaya mountains? convergent transform divergent hot spot
Answers: 1

Physics, 22.06.2019 11:30, rwerjekrryery6750
In order of decreasing light-transmitting capabilities of materials, which is the correct sequence? a. transparent -> translucent -> opaque b. opaque -> transparent -> translucent c. opaque -> translucent -> transparent d. translucent -> transparent -> opaque
Answers: 1
You know the right answer?
Ahelium-filled balloon is launched when the temperature at ground level is 27.8°c and the barometer...
Questions in other subjects:



Biology, 21.05.2020 04:59