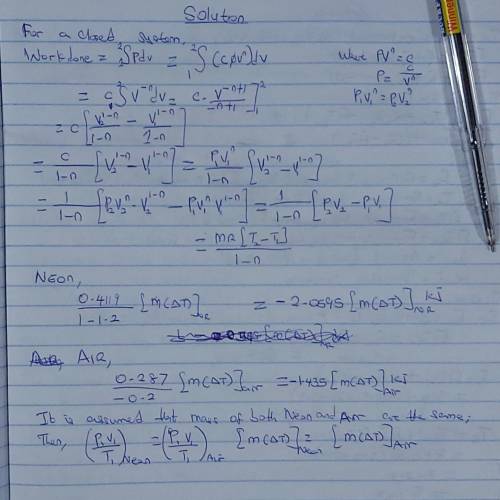Engineering, 07.03.2020 06:26 bain80
Two gases—neon and air—are expanded from P1 to P2 in a closed-system polytropic process with n = 1.2. produces more work when expanded. The gas constants for neon and air are R = 0.4119 and 0.287 kJ/kg·K, respectively.

Answers: 1


Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, brooklyn674
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10, redrosesxx
Water at 55c flows across a flat plate whose surface temperature is held constant at 95c. if the temperature gradient at the plate's surface for a given value of x is 18 c/mm, find a) local heat transfer coefficient. b) heat flux
Answers: 3

Engineering, 04.07.2019 18:20, xcapo1x
Refrigerant-134a enters the compressor of a refrigerator as superheated vapor at 0.14 mpa and -10°c at a rate of 0.05 ka/s and leaves at 0.8 mpa and 50°c. the refrigerant is cooied in the condenser to 0.72 mpa and 26'c. it is then throttled to 0.15 mpa. sketch the t-s diagram for the system and evaluate: 6) the rate of heat removai from the refrigerated space (kw), it) the power input to the compressor (kw), ii) the isentropic efficiency of the compressor (%), and iv) the cop of the refrigerator.
Answers: 2

Engineering, 04.07.2019 18:20, jessie8022
Apiston-cylinder device contains 0.1 m3 of liquid water and 0.9 m3 of water vapor in equilibrium at 800 kpa. heat is transferred at constant pressure until the temperature of water reaches 350 °c. determine (a) the quality of water at the initial state (b) the work associated with this process, (c) the heat associated with this process.
Answers: 2
You know the right answer?
Two gases—neon and air—are expanded from P1 to P2 in a closed-system polytropic process with n = 1.2...
Questions in other subjects:

History, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00





Mathematics, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00

Mathematics, 14.07.2019 02:00