
Chemistry, 21.07.2019 15:00 Dmoney7784
Give the percent yield when 28.16 g of co2 are formed from the reaction of 4.000 moles of c8h18 with 4.000 moles of o2. 2 c8h18(l) + 25 o2(g) → 16 co2(g) + 18 h2o(g) molar mass co2 = 44.01 g/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 20:40, ohgeezy
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Give the percent yield when 28.16 g of co2 are formed from the reaction of 4.000 moles of c8h18 with...
Questions in other subjects:



History, 23.11.2019 03:31

Geography, 23.11.2019 03:31

Biology, 23.11.2019 03:31

Mathematics, 23.11.2019 03:31


Chemistry, 23.11.2019 03:31

Geography, 23.11.2019 03:31

History, 23.11.2019 03:31

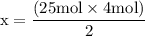 x = 50 moles of oxygen or O₂.
x = 50 moles of oxygen or O₂. 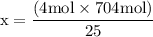 x = 112.64 gram of carbon dioxide
x = 112.64 gram of carbon dioxide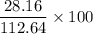 Percentage yield = 25%
Percentage yield = 25%

