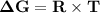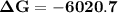Chemistry, 25.07.2019 07:00 zetrenne73
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol. calculate δg at 298 k if the partial pressures of no2 and n2o4 are 0.38atm and 1.64atm , respectively.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:50, hannahmyung1113
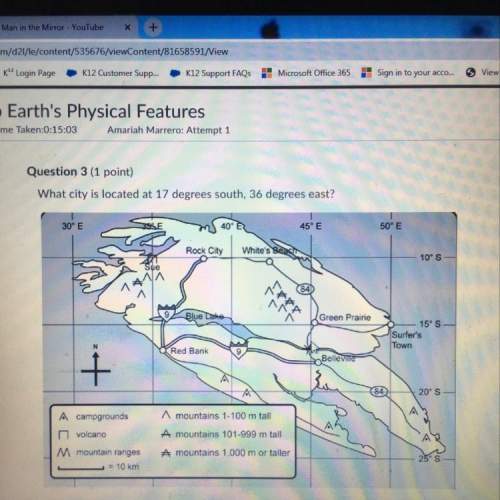
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol....
Questions in other subjects:



History, 30.11.2020 20:30

Mathematics, 30.11.2020 20:30



Mathematics, 30.11.2020 20:30

English, 30.11.2020 20:30