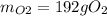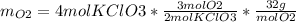The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info: mm o2 = 32 g/mol mm kcl = 74.55 g/mol mm kclo3 = 122.55 g/mol if 4.00 moles of kclo3 are totally consumed, how many grams of oxygen gas would be produced? 192 g 6.00 g 85.3 g 735 g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, andaws21
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1
You know the right answer?
The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info:...
Questions in other subjects:


Arts, 31.01.2020 06:46



Mathematics, 31.01.2020 06:46

History, 31.01.2020 06:46


Biology, 31.01.2020 06:46

Biology, 31.01.2020 06:46