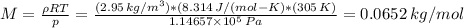Chemistry, 13.07.2019 19:00 KassandraVillegas
What is the molar mass of a pure gaseous compound having a density of 2.95 g/l at 32⁰c and 860 mm hg? (1 atm = 760 mm hg)?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
What is the molar mass of a pure gaseous compound having a density of 2.95 g/l at 32⁰c and 860 mm hg...
Questions in other subjects:

Mathematics, 27.06.2019 08:30

History, 27.06.2019 08:30


Mathematics, 27.06.2019 08:30

History, 27.06.2019 08:30

Mathematics, 27.06.2019 08:30




Social Studies, 27.06.2019 08:30