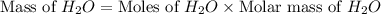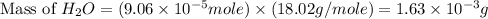Chemistry, 03.07.2019 05:00 rose782751
2h2 +o2 → 2h2o what mass of water forms when 1.45 × 10-3 g o2 react completely? (molar mass of o2 = 32.00 g/mol; molar mass of h2o = 18.02 g/mol) 1.63 × 10-3 g 8.16 × 10-4 g 1.29 × 10-3 g

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
2h2 +o2 → 2h2o what mass of water forms when 1.45 × 10-3 g o2 react completely? (molar mass of o2 =...
Questions in other subjects:




History, 14.04.2020 23:35



Mathematics, 14.04.2020 23:36

Mathematics, 14.04.2020 23:36


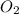 .
.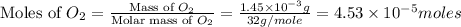
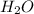 .
.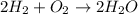
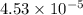 moles of
moles of 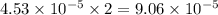 moles of
moles of