
Chemistry, 11.02.2022 14:00 kayranicole1
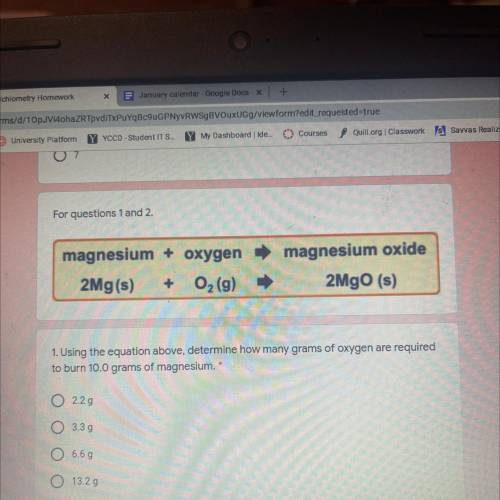
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equation above, determine how many grams of oxygen are required
to burn 10.0 grams of magnesium.
2.29
O 3.39
6.69
Ο Ο
13.29

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, viktoria1198zz
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1


Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equatio...
magnesium oxide
2MgO (s)
1. Using the equatio...
Questions in other subjects:










Biology, 10.12.2019 06:31




