
Chemistry, 16.12.2021 01:20 breanastone15
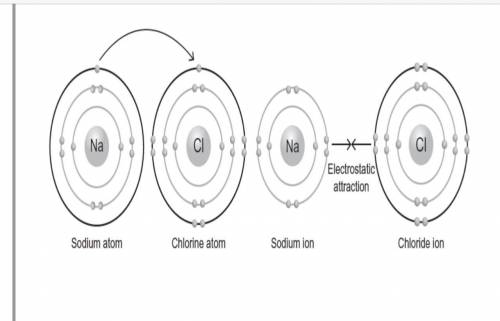
The following figure shows the bond formation of sodium chloride, which is the compound commonly known as table salt.
Which of the following statements are true once the sodium chloride bond formation shown in the figure takes place? Select all that apply.
A. The resulting sodium cation has a stable octet in its valence shell.
B. The resulting chlorine anion has a stable octet in its valence shell.
C. The electrostatic attraction between the positive sodium ion and negative chlorine ion forms a nonpolar bond.
D. The electrostatic attraction between the positive sodium ion and negative chlorine ion forms a polar covalent bond.
E. The electrostatic attraction between the positive sodium ion and negative chlorine ion forms an ionic bond.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
The following figure shows the bond formation of sodium chloride, which is the compound commonly kno...
Questions in other subjects:





Mathematics, 10.07.2019 01:20





Social Studies, 10.07.2019 01:30



