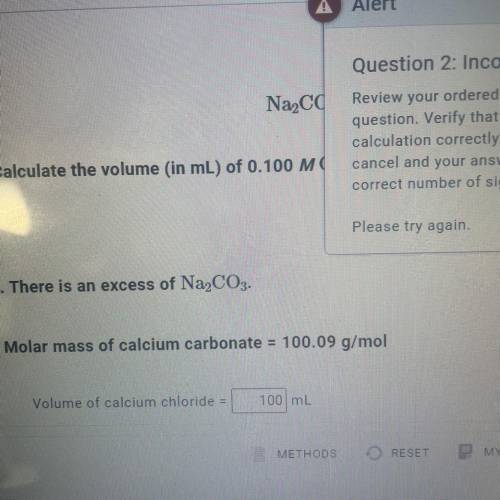
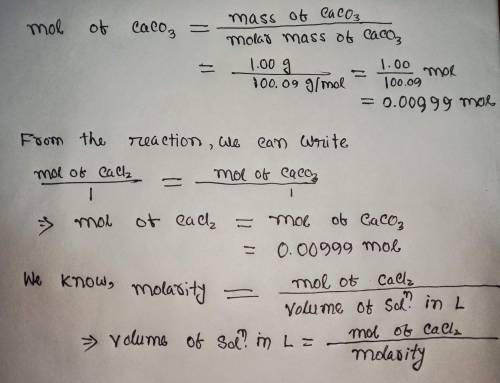Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There...

Chemistry, 28.09.2021 03:10 hammackkatelyn60
Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There is an excess of CaCl2.
Molar mass of calcium carbonate = 100.09 g/mol
*The answer is not 100ml or 10ml. Somehow the rounding up is not working well.*

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
You know the right answer?
Questions in other subjects:



History, 08.04.2021 21:50

Mathematics, 08.04.2021 21:50

Mathematics, 08.04.2021 21:50

Mathematics, 08.04.2021 21:50