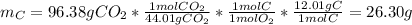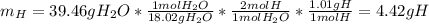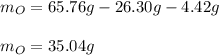A compound contains only carbon, hydrogen, and oxygen. Combustion of 65.76 g of the compound yields 96.38 g of CO2 and 39.46 g of H2O.
The molar mass of the compound is 90.078 g/mol.
1. Calculate the grams of carbon (C) in 65.76 g of the compound:
2. Calculate the grams of hydrogen (H) in 65.76 g of the compound.
3. Calculate the grams of oxygen (O) in 65.76 g of the compound.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

You know the right answer?
A compound contains only carbon, hydrogen, and oxygen. Combustion of 65.76 g of the compound yields...
Questions in other subjects:


Chemistry, 01.05.2022 21:50

Mathematics, 01.05.2022 21:50




Mathematics, 01.05.2022 22:40


Mathematics, 01.05.2022 22:50

SAT, 01.05.2022 22:50