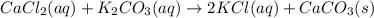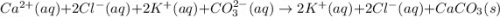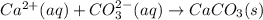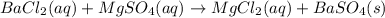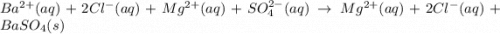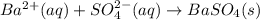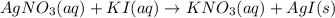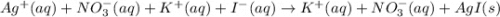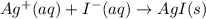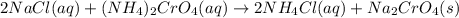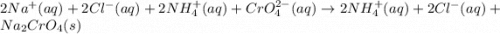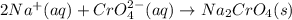Chemistry, 03.06.2021 23:40 mirandac6775
Show the complete ionic equation and net ionic equation for all the equations below, then state whether or not a precipitate (insoluble compound) will form. To receive full credit, you must show ALL your work.
Cacl2(aq) + K2co3(aq) + >
Bacl2(aq) + MgSO4(aq) + >
AgNO3(aq) + Kl(aq) →
Nacl(aq) + (NH4)2Cro4(aq) →

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1
You know the right answer?
Show the complete ionic equation and net ionic equation for all the equations below, then state whet...
Questions in other subjects:



Business, 20.10.2020 20:01



Health, 20.10.2020 20:01

Physics, 20.10.2020 20:01

Geography, 20.10.2020 20:01