

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
0.90 g of hydrogen chloride (HCl) is dissolved in water to make 2.0 L of solution. What is the pH of...
Questions in other subjects:


Mathematics, 31.01.2021 06:20


Chemistry, 31.01.2021 06:20


Advanced Placement (AP), 31.01.2021 06:20



History, 31.01.2021 06:20

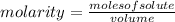
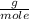 , then the following rule of three can be applied: if there are 35.45 grams of HCl in 1 mole, 0.9 grams of HCl in how many moles will they be?
, then the following rule of three can be applied: if there are 35.45 grams of HCl in 1 mole, 0.9 grams of HCl in how many moles will they be?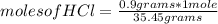
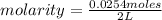
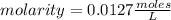
 . So [HCl] = [H⁺]
= 0.0127
. So [HCl] = [H⁺]
= 0.0127 


