
Chemistry, 08.05.2021 08:10 sparky1234
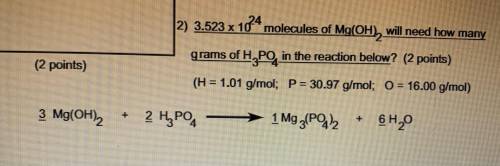
3.523 x 10^24 molecules of Mg(OH2) will need how many grams of H3PO4 in the reaction below
H=1.01 g/mol
P=30.97 g/mol
O=16.00 g/mol
3 Mg(OH)2 + 2 H3PO4 -> 1 Mg3 (PO4 )2 + 6 H2O

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:00, scottykinkade7860
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
3.523 x 10^24 molecules of Mg(OH2) will need how many grams of H3PO4 in the reaction below
H=1.01...
Questions in other subjects:


Mathematics, 25.01.2021 01:00


English, 25.01.2021 01:00


Physics, 25.01.2021 01:00

Mathematics, 25.01.2021 01:00





