
Chemistry, 03.05.2021 19:20 emmmmmmaaaa
For this reaction: 6 PbO + O2 → 2 Pb304 a. How many moles of Pb304 are produced from 1.25 moles of oxygen?
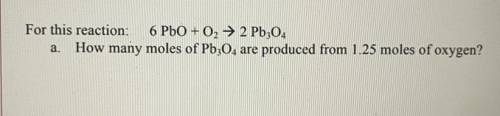

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1


Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
For this reaction: 6 PbO + O2 → 2 Pb304
a. How many moles of Pb304 are produced from 1.25 moles of...
Questions in other subjects:





Mathematics, 23.03.2020 19:56



Mathematics, 23.03.2020 19:56

Mathematics, 23.03.2020 19:56



