
Chemistry, 29.04.2021 01:30 RiceLord9562
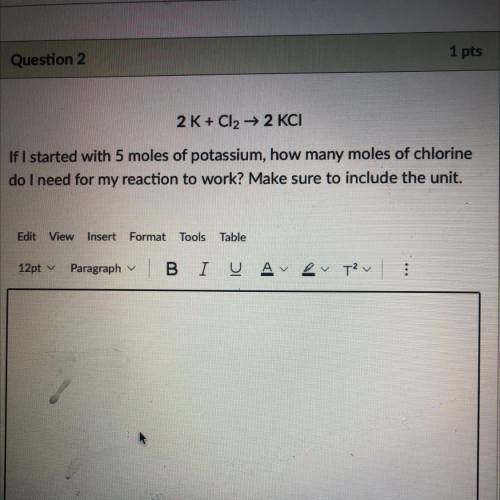
if i started with 5 moles of potassium, how many moles of chlorine do i need for my reaction to work? make sure to include the unit. marking brainliest help pls

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 08:20, debramknoxx
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
if i started with 5 moles of potassium, how many moles of chlorine do i need for my reaction to work...
Questions in other subjects:





History, 19.07.2019 09:00

Mathematics, 19.07.2019 09:00


German, 19.07.2019 09:00


Physics, 19.07.2019 09:00



