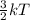Chemistry, 15.01.2020 01:31 braydentillery1221
Asample of gas is enclosed in a container of fixed volume. identify which of the following statements are true. check all that apply. if the container is heated, the gas particles will lose kinetic energy and temperature will increase.
if the container is heated, the gas particles will lose kinetic energy and temperature will increase.
if the container is cooled, the gas particles will lose kinetic energy and temperature will decrease.
if the gas particles move more quickly, they will collide more frequently with the walls of the container and pressure will increase.
if the gas particles move more quickly, they will lose energy more rapidly and collide with the walls of the container less often, and pressure will decrease.
if the gas particles move more quickly, they will collide with the walls of the container more often and with more force, and pressure will increase.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, krystenlitten
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1
You know the right answer?
Asample of gas is enclosed in a container of fixed volume. identify which of the following statement...
Questions in other subjects:

Mathematics, 02.07.2019 07:30

Mathematics, 02.07.2019 07:30

History, 02.07.2019 07:30

History, 02.07.2019 07:30


Mathematics, 02.07.2019 07:30


Biology, 02.07.2019 07:30