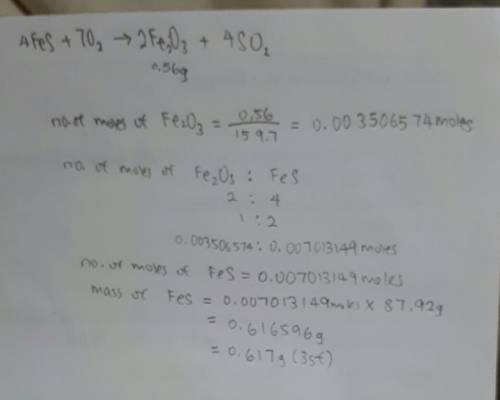In the following reaction, how many grams of ferrous sulfide (fes) will produce
0.56 grams of...


Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, bgarrison364
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 03.04.2021 06:10

Business, 03.04.2021 06:10



Mathematics, 03.04.2021 06:10

Mathematics, 03.04.2021 06:10



French, 03.04.2021 06:10