
Chemistry, 03.10.2019 11:30 JvGaming2001
Astudent requires 2.00 l of 0.100 m nh4no3 from a 1.75 m nh4no3 stock solution. what is the correct way to get the solution?
use mc021-1.jpg.
measure 114 ml of the 1.75 m solution, and dilute it to 1.00 l.
measure 114 ml of the 1.75 m solution, and dilute it to 2.00 l.
measure 8.75 ml of the 1.75 m solution, and dilute it to 2.00 l.
measure 8.75 ml of the 0.100 m solution, and dilute it to 2.00 l.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Astudent requires 2.00 l of 0.100 m nh4no3 from a 1.75 m nh4no3 stock solution. what is the correct...
Questions in other subjects:


Biology, 15.01.2021 18:10

Social Studies, 15.01.2021 18:10


Advanced Placement (AP), 15.01.2021 18:10





Mathematics, 15.01.2021 18:10

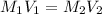
 = molarity of first solution
= molarity of first solution = Volume of first solution
= Volume of first solution = molarity of second solution
= molarity of second solution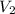 = Volume of second solution
= Volume of second solution



