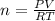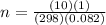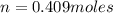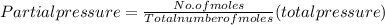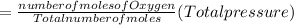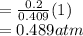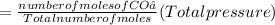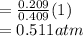Chemistry, 13.10.2019 23:30 jasminelynn135owmyj1
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement is true for the partial pressures of o2 and co2 if 0.2 mole of o2 is present in the flask? (given the universal gas constant r = 0.082 l∙atm/k∙mol)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement i...
Questions in other subjects:

Chemistry, 17.07.2020 20:01



Mathematics, 17.07.2020 20:01