Chemistry, 19.04.2021 16:10 Albertrami2251
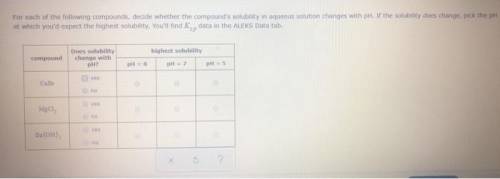
CaCO3 For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find data in the ALEKS Data tab. compound Does solubility change with pH? highest solubility pH = 5 pH = 6 pH = 7 yes no yes no yes no g

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
CaCO3 For each of the following compounds, decide whether the compound's solubility in aqueous solut...
Questions in other subjects:


Social Studies, 19.09.2021 14:00


Mathematics, 19.09.2021 14:00

History, 19.09.2021 14:00

Mathematics, 19.09.2021 14:00

Mathematics, 19.09.2021 14:00


Mathematics, 19.09.2021 14:00