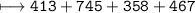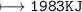Use the reaction and bond information to answer the question.
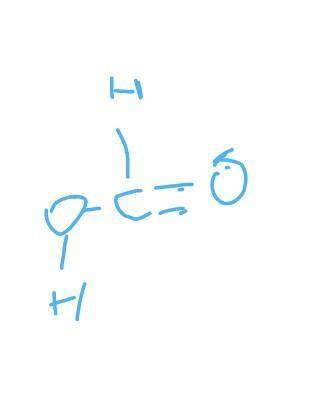
H2 + CO2 → CH2O2
Reactan...

Chemistry, 19.09.2021 14:00 CuteDoggo1828
Use the reaction and bond information to answer the question.
H2 + CO2 → CH2O2
Reactant bond energies: H–H is 432 kJ/mol, C=O is 799 kJ/mol
Product bond energies:
C–H is 413 kJ/mol, C=O is 745 kJ/mol
C–O is 358 kJ/mol, O–H is 467 kJ/mol
How much energy will be given off by the products?
1,570 kJ
1,983 kJ
1,238 kJ
1,516 kJ

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1


Chemistry, 23.06.2019 05:30, brianrodriguez2005
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b. cns c. ans d. pns
Answers: 1

Chemistry, 23.06.2019 13:30, leianagaming
1. what is boyle’s law? • state the definition of the law in words. • what are the assumptions of boyle’s law? • write at least one mathematical equation that represents the law. • what can be calculated with boyle’s law? • using a gas-filled balloon as an example, describe what is happening to the gas molecules inside the balloon before and after you squeeze it.
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 28.06.2019 06:00

Mathematics, 28.06.2019 06:00

Biology, 28.06.2019 06:00


Geography, 28.06.2019 06:00

Mathematics, 28.06.2019 06:00

History, 28.06.2019 06:00

Mathematics, 28.06.2019 06:00


English, 28.06.2019 06:00