
Chemistry, 03.02.2020 23:00 saintsfan2004
If 62.0 grams of magnesium metal (mg) react with 55.5 grams of oxygen gas (o2) in a synthesis reaction, how many grams of the excess reactant will be left over when the reaction is complete?
be sure to write out the balanced equation for this reaction and to show all of your work.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
If 62.0 grams of magnesium metal (mg) react with 55.5 grams of oxygen gas (o2) in a synthesis reacti...
Questions in other subjects:


Mathematics, 15.02.2021 20:20

Biology, 15.02.2021 20:20


Mathematics, 15.02.2021 20:20





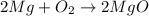
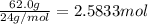
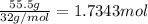
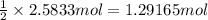 of oxygen gas.
of oxygen gas.

