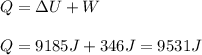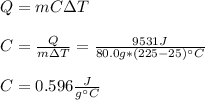Chemistry, 18.03.2021 02:50 genyjoannerubiera
An 80.0 g sample of a gas was heated from 25 °C to 225 °C. During this process, 346 J of work was done by the system and its internal energy increased by 9185 J. What is the specific heat of the gas?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
An 80.0 g sample of a gas was heated from 25 °C to 225 °C. During this process, 346 J of work was do...
Questions in other subjects: