
Chemistry, 20.12.2019 23:31 michaelchavez6959127
In a different experiment, a student places a piece of pure ba(s) in a beaker containing 250.ml of 6.44mhcl(aq) and observes that the rate of appearance of h2(g) bubbles when ba(s) was added is much faster than the rate of appearance of bubbles when the mg(s) was added. the student claims that the greater reactivity is due to the difference in ionization energy between mg and ba. explain the difference in ionization energy between mg and ba in terms of atomic structure.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, steven2996
What can be the use of smoke transformed into liquid?
Answers: 1

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

You know the right answer?
In a different experiment, a student places a piece of pure ba(s) in a beaker containing 250.ml of 6...
Questions in other subjects:


Mathematics, 29.09.2020 04:01


Physics, 29.09.2020 04:01

Mathematics, 29.09.2020 04:01


Social Studies, 29.09.2020 04:01



Physics, 29.09.2020 04:01

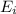
![[Ne]3s^2](/tpl/images/0428/3026/d3dcb.png)
![[Xe]6s^2](/tpl/images/0428/3026/f6f70.png)


