
Chemistry, 25.02.2021 19:20 tyairamifflin2411
Calculations:
1. The actual value for absolute zero in degrees Celsius is –273.15. Use the formula below
to determine your percent error for both gas samples.
Jexperimental value - actual valuel x 100
actual value
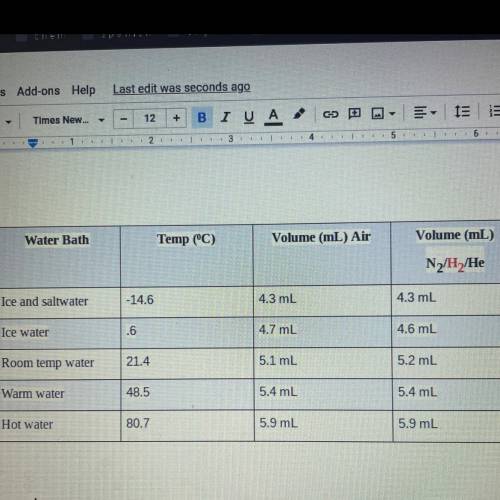
2. If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in
each syringe? (Hint: Choose one volume and temperature pair from your data table to use
in your ideal gas law calculation.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 21.06.2019 17:10, PSBSolarYT
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1
You know the right answer?
Calculations:
1. The actual value for absolute zero in degrees Celsius is –273.15. Use the formula...
Questions in other subjects:



Physics, 24.11.2020 21:50

Business, 24.11.2020 21:50

Health, 24.11.2020 21:50

Mathematics, 24.11.2020 21:50




Biology, 24.11.2020 21:50



