
Chemistry, 19.02.2021 22:30 yaneiryx5476
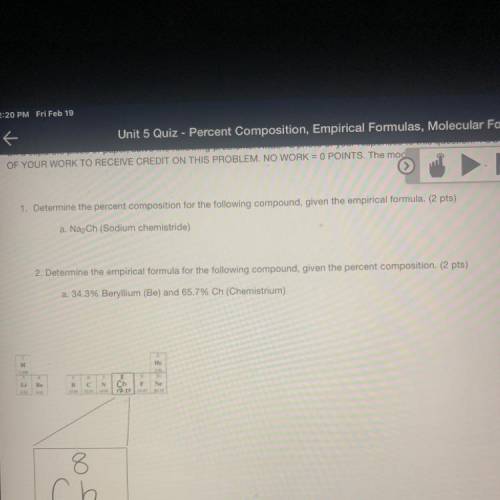
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts)
a. Na2Ch (Sodium chemistride)
2. Determine the empirical formula for the following compound, given the percent composition. (2 pts)
a. 34.3% Beryllium (Be) and 65.7% Ch (Chemistrium)
H
Не
Be
B
c С
N
Ch F Ne
17.250 30
8
ch
Chemistrium
17.25

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3



Chemistry, 22.06.2019 23:50, datboyjulio21
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts...
Questions in other subjects:

Mathematics, 19.10.2019 00:10






Chemistry, 19.10.2019 00:20





