
Chemistry, 17.01.2021 18:40 paulitaaustin
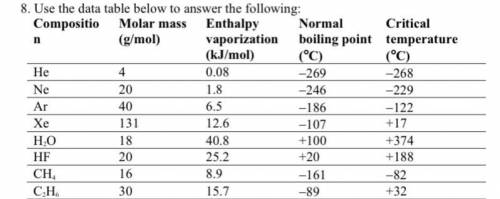
A. Among nonpolar liquids, those with higher molar masses tend to have normal boiling points that are (higher, lower, or about the same).
b. Among compounds of approximately the same molar mass, those with greater polarities tend to have enthalpies of vaporization that are (higher, lower, or about the same).
c. Which is the only noble gas listed that is stable as a liquid at 0°C? Explain your answer using the concept of critical temperature.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
A. Among nonpolar liquids, those with higher molar masses tend to have normal boiling points that ar...
Questions in other subjects:






History, 31.01.2020 18:55

Physics, 31.01.2020 18:55



History, 31.01.2020 18:55



