
Chemistry, 15.01.2021 01:40 rleiphart1
A hydrogen atom absorbs a photon radiation causing an electron to jump from level one to level two. If the photon/radiation has a wavelength of 5.62 x 10^-3 m to calculate the frequency of the radiation absorbed and the energy change of the electron
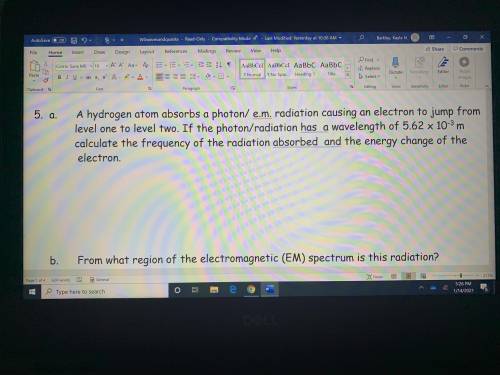

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, HHHHHHHHHMMMMMMMMM
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
A hydrogen atom absorbs a photon radiation causing an electron to jump from level one to level two....
Questions in other subjects:







Biology, 11.11.2020 18:10

Mathematics, 11.11.2020 18:10

Mathematics, 11.11.2020 18:10

Social Studies, 11.11.2020 18:10



