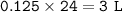Chemistry, 27.12.2020 03:10 makaylapink8167
3. The equation for the decomposition of sodium hydrogencarbonate is :
2 NaHCO3 (s) → Na2CO3 (s) + H2O(l) + CO2(8)
If 21 g of sodium hydrogencarbonate were decomposed, calculate
(a) the mass of the residue formed.
(b) the volume of carbon dioxide evolved measured at r. t.p.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
3. The equation for the decomposition of sodium hydrogencarbonate is :
2 NaHCO3 (s) → Na2CO3 (s) +...
Questions in other subjects:



Mathematics, 30.01.2022 14:00


Mathematics, 30.01.2022 14:00




Mathematics, 30.01.2022 14:00