
Chemistry, 15.12.2020 22:20 kendramiller3965
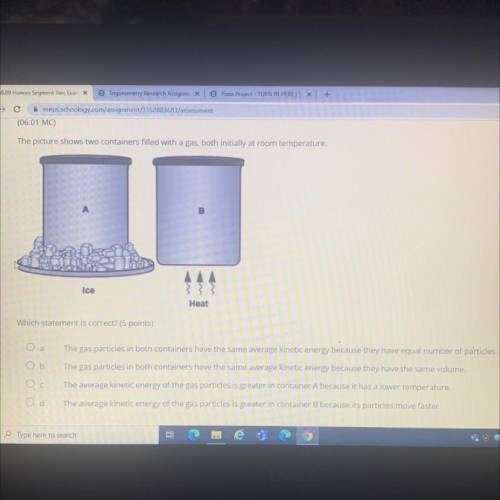
The picture shows two containers filled with a gas, both initially at room temperature.
B
Ice
Heat
Which statement is correct? (5 points)
О а
The gas particles in both containers have the same average kinetic energy because they have equal number of particles.
Ob
The gas particles in both containers have the same average kinetic energy because they have the same volume.
Ос
The average kinetic energy of the gas particles is greater in container A because it has a lower temperature.
The average kinetic energy of the gas particles is greater in container B because its particles move faster.
Od

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1


Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1
You know the right answer?
The picture shows two containers filled with a gas, both initially at room temperature.
B
Ice...
Ice...
Questions in other subjects:

Social Studies, 22.11.2020 14:10



Mathematics, 22.11.2020 14:10

English, 22.11.2020 14:10


History, 22.11.2020 14:10


Geography, 22.11.2020 14:10



