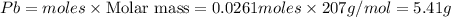You dissolve 8.65 grams of lead(II) nitrate in water, and then you add 2.50 grams of aluminum. This reaction occurs: 2Al(s) + 3Pb(NO3)2(aq) → 3Pb(s) + 2Al(NO3)3(aq). What’s the theoretical yield of solid lead? Use the ideal gas resource and the periodic table. A. 5.41 g B. 11.2 g C. 19.2 g D. 28.8 g Reset

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2


Chemistry, 23.06.2019 16:30, jules8022
Boron has an average atomic mass of 10.81. one isotope of boron has a mass of 10.012938 and a relative abundance of 19.80 percent . the other isotope has a relative abundance of 80.20 percent what is the mass of that isotope? report two decimal places
Answers: 1
You know the right answer?
You dissolve 8.65 grams of lead(II) nitrate in water, and then you add 2.50 grams of aluminum. This...
Questions in other subjects:





Biology, 12.01.2021 01:50



Mathematics, 12.01.2021 01:50


Chemistry, 12.01.2021 01:50

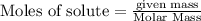
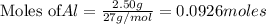
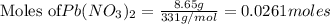
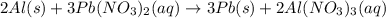
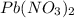 require = 2 moles of
require = 2 moles of 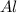
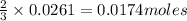 of
of 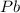
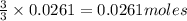 of
of