
Chemistry, 10.12.2020 08:40 jademckinziemea
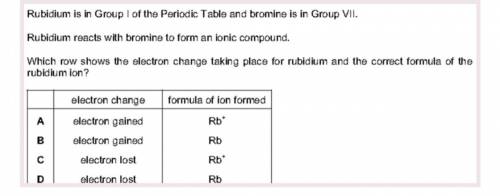
Rubidium is in Group I of the Periodic Table and bromine is in Group VII.
Rubidium reacts with bromine to form an ionic compound.
Which row shows the electron change taking place for rubidium and the correct formula of the
rubidium ion?
formula of ion formed
A
electron change
electron gained
electron gained
Rb
B
Rb
C
electron lost
Rb
D
electron lost
Rb

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
Rubidium is in Group I of the Periodic Table and bromine is in Group VII.
Rubidium reacts with brom...
Questions in other subjects:

History, 03.02.2020 12:46



Mathematics, 03.02.2020 12:46

Chemistry, 03.02.2020 12:46


Mathematics, 03.02.2020 12:46


Mathematics, 03.02.2020 12:46

History, 03.02.2020 12:46



