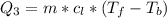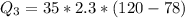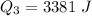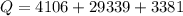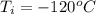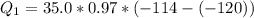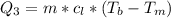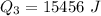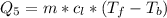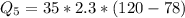Ethanol (C2H5OH) melts at –114 °C and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpy of vaporization is 38.56 kJ/mol. The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K, respectively. The average specific heat of gaseous ethanol is about 1.80 J/g-K. a. How much heat is required to convert 35.0 g of ethanol at 27 °C to the vapor phase at 120 °C? b. How much heat is required to convert the same amount of ethanol at –120 °C to the vapor phase at 120 °C?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2


Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Ethanol (C2H5OH) melts at –114 °C and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kJ/m...
Questions in other subjects:

Social Studies, 07.09.2021 16:30

Business, 07.09.2021 16:30

Biology, 07.09.2021 16:30


Computers and Technology, 07.09.2021 16:30


Social Studies, 07.09.2021 16:40



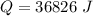





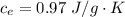
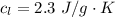
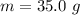
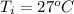
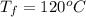
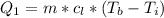
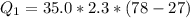
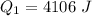
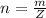

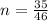
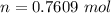



 is mathematically represented as
is mathematically represented as