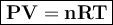Chemistry, 23.11.2020 08:00 brookemcelhaney
0.817 grams of an unknown gas occupies 1.25 L at 27.0 C and a pressure of 765
mm Hg. Is the gas most likely methane (CH 4 ), ethane (C 2 H 6 ), propane or butane?
Show work to support your answer.

Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
0.817 grams of an unknown gas occupies 1.25 L at 27.0 C and a pressure of 765
mm Hg. Is the gas mo...
Questions in other subjects:




Mathematics, 30.04.2021 14:00


Social Studies, 30.04.2021 14:00



Biology, 30.04.2021 14:00