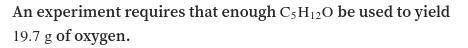Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, 20jhuffman
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
An experiment requires that enough C3H8O be used to yield of oxygen .
How much C3H8O must be weighe...
Questions in other subjects:



History, 26.03.2020 05:04







English, 26.03.2020 05:05