
Chemistry, 16.10.2020 18:01 Clover1072
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tablet. The chlorate is titrated with iodide ions until the end point. The student reported that 29.50 mL of 0.100 M KI solution was required to reach the end point of a titration when 10 allergy tablets containing chlorate as the main active ingredient are dissolved in 25.00 mL of distilled water. What mass in grams of chlorate is present in the 10 allergy tablets?
Use the Balanced chemical equation to answer the question.
6, 1, 6, 3, 1, 3
Hint: this is a net ionic equation, so spectator ions have been removed!
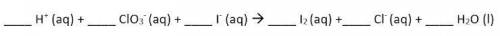

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2


Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1
You know the right answer?
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tabl...
Questions in other subjects:






History, 05.10.2019 20:40


Advanced Placement (AP), 05.10.2019 20:40


Social Studies, 05.10.2019 20:40



