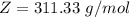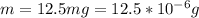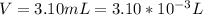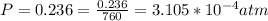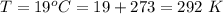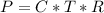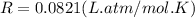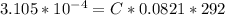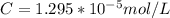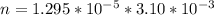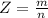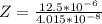Working on-board a research vessel somewhere at sea, you have (carefully) isolated 12.5 micrograms (12.5 ×10–6 g) of what you hope is pure saxitoxin (a non-electrolyte) from a poisonous (and quite cross) puffer fish. You dissolve this sample in 3.10 mL of water and determine that the osmotic pressure of the resulting solution is 0.236 torr at 19ºC (760 torr = 1.00 atm). What is the molar mass of the compound?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
Working on-board a research vessel somewhere at sea, you have (carefully) isolated 12.5 micrograms (...
Questions in other subjects: