
Chemistry, 12.08.2020 05:01 peytonwilson2003
A saturated sodium carbonate solution at 0°C contains 7.1 g of dissolved sodium carbonate per 100. mL of solution. The solubility product constant for sodium carbonate at this temperature is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 08:00, mackaylabarnes22
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
A saturated sodium carbonate solution at 0°C contains 7.1 g of dissolved sodium carbonate per 100. m...
Questions in other subjects:










Mathematics, 02.07.2019 03:10

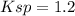
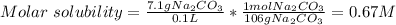
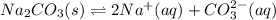
![Ksp=[Na^+]^2[CO_3^{2-}]](/tpl/images/0718/5031/3f46d.png)




