but know think of it in terms of bond breaking and bond making.
h–h + h–h + o=o ==> h–o–h + h–o–h
as you can see from the diagram, theoretically, more energy is released by forming water, than was absorbed in breaking up the original hydrogen and oxygen molecules.
the energy level of the fuel + oxygen is higher than that of water.
therefore the fuel + oxygen mixture has the potential to release potential chemical energy as heat energy or, crucially, electrical energy, if the reaction can be made to go via oxidation and reduction reactions involving electron transfer.
see this page for more details of the full bond energy calculations
a fuel cell is a device to make electricity that functions like an electrical cell or battery that you supply with a fuel (liquid or gas) and an oxidant (oxidising agent, usually oxygen gas) which react together in a redox reaction (oxidation + reduction) to release energy as a flow of electrons i.e. an electrical current capable of doing useful work.
the oxidation of the fuel and the reduction of the oxidant reactions take place on electrodes (see diagram on right, all fully explained further down the page).
fuel cells essentially a device to release the chemical potential energy of combustible fuels as electrical energy at a much lower temperature and without the flame!
so, the idea of a hydrogen–oxygen fuel cell is to release the energy from hydrogen reacting with oxygen, not as heat as in normal combustion in air, but as useful electrical energy i.e. a practical electricity supply.
hydrogen-oxygen fuel cells are much efficient than conventional power stations or batteries (e.g. zinc-carbon) because the electrical energy is directly generated from the chemical reaction between the oxidant and the fuel - there are no complications with boilers, turbines and generators.
in fossil fuel power stations, motor vehicles, coal/gas fires etc. quite a percentage of energy is lost as waste heat.
with a fuel cell there are fewer stages in producing the useful energy, so there is less opportunity to lose potentially useful energy e.g. waste heat, friction from moving parts etc.
a hydrogen–oxygen fuel cell is a non–polluting clean fuel since the only combustion product is water.
fuel cells do not produce pollutants like carbon monoxide, sulfur dioxide and nitrogen oxides - gases you get from fossil fuel combustion in power stations or burnt road vehicles..
cars powered by fuel cells would be quite an environmental advantage in cities, where electric cars are already beginning to be significantly used in developed countries.
fuel cells could replace larger batteries which are not easily recycled and contain highly toxic metal compounds.
it would be an ideal fuel on this basis e.g. for motor vehicles, but that's not the only factor to consider!
it would be ideal if the hydrogen fuel could be manufactured by electrolysis of water e.g. using solar cells.
hydrogen can be used to power fuel cells.
it all sounds wonderful but, still technological problems to solve for large scale manufacture and distribution of 'clean' hydrogen gas or use in generating electricity and its rather an inflammable explosive gas!
explanation:
fuel cells e.g. the hydrogen-oxygen fuel electrical cell
fuel cells have to be supplied by an external source of fuel (e.g. hydrogen) and an oxidant e.g. oxygen or air.
the hydrogen or any other fuel is oxidised electrochemically inside the fuel cell to produce a potential difference i.e. a voltage capable of producing a working current.
the overall chemical reaction in a hydrogen fuel electrochemical cell involves the oxidation of hydrogen by oxygen to produce only water.
hydrogen fuel cells offer an alternative to rechargeable cells and batteries.
a fuel cell will produce a potential difference ('voltage') and a workable electric current until one of the reactants is used up.
hydrogen gas can be used as fuel.
it burns with a pale blue flame in air reacting with oxygen to be oxidised to form water.
hydrogen + oxygen ==> water
2h2(g) + o2(g) ==> 2h2o(l)
















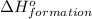 for the reaction is 591.9 kJ.
for the reaction is 591.9 kJ.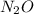 is:
is: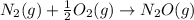
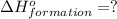
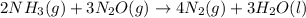
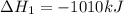 ( ÷ 3)
( ÷ 3)![4NH_3(g)+3O_2(g)\rightarrow 2N_2(g)+6H_2O(l) [tex]\Delta H_2=1531kJ](/tpl/images/0041/0429/f3925.png) ( ÷ 6)
( ÷ 6)![\Delta H^o_{formation}=[\frac{\Delta H_1}{3}]+[\frac{\Delta H_2}{6}]](/tpl/images/0041/0429/5c537.png)
![\Delta H^o_{formation}=[\frac{1010}{3}]+[\frac{1531}{6}]\\\\\Delta H^o_{formation}=591.9kJ](/tpl/images/0041/0429/fba80.png)


