Chemistry, 05.07.2020 23:01 stefancvorovic1
How many grams of H 2O are produced from 28.8 g of O 2? (Molar Mass of H 2O = 18.02 g) (Molar Mass of O 2=32.00 g) 4 NH 3 (g) + 7 O 2 (g) → 4 NO 2 (g) + 6 H 2O (g)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
How many grams of H 2O are produced from 28.8 g of O 2? (Molar Mass of H 2O = 18.02 g) (Molar Mass o...
Questions in other subjects:

Chemistry, 04.01.2020 03:31


Biology, 04.01.2020 03:31







 will be produced from the given mass of oxygen
will be produced from the given mass of oxygen
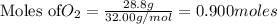
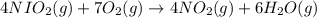
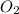 produce = 6 moles of
produce = 6 moles of 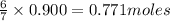 of
of