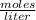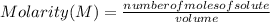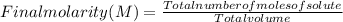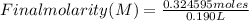Chemistry, 24.06.2020 15:01 jd326677777gfc
8. A 65.0 mL 0.513 mol/l solution of glucose (C6H1206) was mixed with 125.0 mL of
2.33 mol/l glucose solution. What is the molar concentration of the final solution?
Assume the volumes are additive. The molar mass of glucose is 180 g/mol (10
points)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, natannale
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
8. A 65.0 mL 0.513 mol/l solution of glucose (C6H1206) was mixed with 125.0 mL of
2.33 mol/l glucos...
Questions in other subjects: