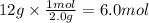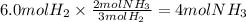1 point
Using the balanced chemical equation; how many moles of ammonia will
be made if...


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 21.01.2021 22:00

Social Studies, 21.01.2021 22:00

Mathematics, 21.01.2021 22:00



Computers and Technology, 21.01.2021 22:00


Computers and Technology, 21.01.2021 22:00